Clinical Testosterone
by Austin Baraki | January 04, 2017
Testosterone is a steroid hormone that exerts a wide number of important androgenic and anabolic effects in the human body. It is a fundamental component of male physiology, although smaller amounts are produced in females as well. Adequate levels are crucial for optimal health, and there have been growing concerns about hypogonadism (commonly known as “Low T”) being a highly prevalent and under-diagnosed condition with the potential to dramatically reduce quality of life among males.
Among aging individuals and those interested in strength training, optimizing testosterone levels is of even greater concern. In addition to improving quality of life, people want to ensure they get the best return on their investment of time and effort in training – particularly those at risk for anabolic resistance and sarcopenia. We frequently get questions about testosterone levels and testosterone replacement therapy, so a review of the topic is both worthwhile and overdue.
Let’s begin with a basic introduction into the pertinent components of the endocrine system. Although things may seem complicated and you might encounter a lot of new vocabulary, an understanding of these components and their interactions is essential to grasp how clinical decisions are made in the evaluation and treatment of hypogonadism.
The Hypothalamic-Pituitary-Gonadal Axis
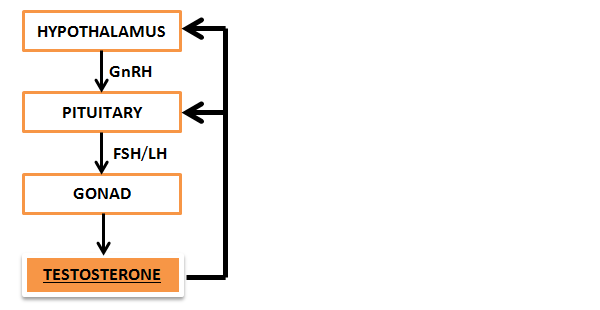
The Hypothalamus is an area deep inside the brain responsible for regulating a vast number of physiologic processes and systems including the autonomic nervous system, the endocrine system, body temperature, appetite, fatigue, and circadian rhythm, among many others. Most pertinent to today’s discussion is its intermittent, pulsatile release of a particular hormone known as Gonadotropin-Releasing Hormone (GnRH).
Bursts of GnRH travel down to the Pituitary gland, an endocrine organ located in the brain just below the hypothalamus. The pituitary gland secretes a number of hormones, but GnRH specifically stimulates the release of the Gonadotropins: Follicle-stimulating Hormone (FSH) and Luteinizing Hormone (LH). These synergistic hormones subsequently act on the testes to stimulate sperm and testosterone production, respectively.
This three-tiered system is known as the Hypothalamic-Pituitary-Gonadal (HPG) axis, and these three components communicate through feedback mechanisms to maintain homeostasis. If blood levels of free testosterone decrease, the pituitary gland and hypothalamus will “sense” this drop and compensate by releasing more GnRH and LH, stimulating the testes to restore normal levels. However, problems can start to occur when one or more components of the HPG axis become diseased, suppressed, or are otherwise unable to maintain homeostasis.
Testosterone
Testosterone is produced by putting cholesterol through a series of enzymatic conversions in the testes (>95%) with a smaller fraction (<5%) produced in the adrenal glands. It is then released into the bloodstream where, like the majority of systemic hormones, it exists primarily in “protein-bound” form.
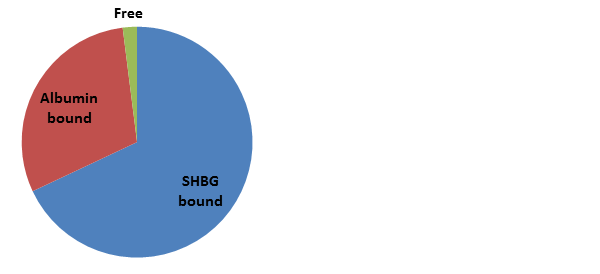
Approximately 60% is tightly bound by Sex-Hormone Binding Globulin (SHBG), about 38% is very loosely bound to Albumin, and about 2% is “free” – and therefore biologically active. Since the albumin-bound fraction is very loosely attached, it is released to the tissues quite easily and is therefore considered part of the pool of “bioavailable" testosterone together with free testosterone.
These protein-bound fractions maintain a protected “buffer” pool of inactive testosterone in dynamic equilibrium with free testosterone. As free testosterone is used up in the tissues (or degraded in the circulation or by the liver), the equilibrium kinetics shift to promote release of this protein-bound testosterone, which helps maintain more consistent free testosterone levels. Without these carrying proteins, the rapid utilization or degradation of free testosterone could result in acute, dramatic fluctuations in serum levels.
Free testosterone primarily exerts its effects by binding the androgen receptor. It exerts both anabolic (i.e., growth) and androgenic (i.e., virilizing) effects depending on the target tissue, and provides feedback to the pituitary and hypothalamus to maintain homeostasis.
Most of testosterone's anabolic effects occur when the activated androgen receptor stimulates gene expression and activates various cell signaling pathways. The primary effects of concern to the strength training population include testosterone’s ability to directly stimulate protein synthesis and growth in muscle and bone, to inhibit fat production and muscle protein breakdown, and to stimulate growth and repair of motor nerves. It should therefore be obvious why testosterone and other anabolic androgens are frequently used by athletes: these effects result in a more efficient neuromuscular system stimulating bigger, stronger muscles to pull on bigger, stronger bones. Testosterone’s numerous additional effects and their mechanistic details could fill several textbooks and are outside the scope of this article.
Certain tissues – such as prostate, hair follicles, and liver – express the enzyme 5-alpha-reductase, which converts free testosterone to dihydrotestosterone (DHT). This hormone is several times more potent than testosterone and is primarily responsible for androgenic effects, including deepening of the voice and growth of body hair, sex organs, and prostate. It also appears to have stimulant effects in the central nervous system and modulates a number of other neurotransmitters that affect mood, aggression, and memory. Paradoxically, excessive local DHT production in hair follicles has been implicated as a factor in male-pattern balding (androgenetic alopecia), and this, along with enlarged prostates and prostate cancer, are commonly treated with 5-alpha-reductase inhibitor medications such as Finasteride (commonly known as Propecia or Proscar).
Other tissues, including adipose (fat), testes, brain, skin, and bone, express the enzyme aromatase, which converts testosterone (but not DHT) to a type of estrogen known as estradiol (E2). Estradiol and related hormones bind the estrogen receptor and exert a number of important effects on the sex organs, brain, skin, liver, and cardiovascular system. Perhaps surprisingly, maintaining normal estrogen levels has been shown to prevent muscular atrophy and to support the satellite cells needed for muscle hypertrophy.
Estrogen clearly has some useful properties and is not inherently an "enemy" to be eradicated from the male body. However, both inadequate and excess estrogen levels are associated with problems such as osteoporosis, gynecomastia, cardiovascular complications, and several types of cancers. High estrogen levels often result from obesity, in which the aromatase expressed in excess body fat converts free testosterone into estrogen. It can also result from excessive use of exogenous testosterone, where supraphysiologic levels result in aromatization as well.
Circulation and Natural Variation
Serum testosterone levels vary from hour to hour and day to day depending on four primary factors: 1) rate of production, 2) rate of interconversion, 3) rate of clearance, and 4) the serum concentration of SHBG. Given all these factors and the volatility of serum levels, multiple measurements are needed in order to confirm a diagnosis of “low testosterone”.
Testosterone levels also follow a diurnal rhythm, typically peaking in the early morning between 8-10 AM (assuming a normal night’s sleep) and subsequently fall through the rest of the day. This circadian rhythm is generally lost with aging, and levels are a bit more stable between morning and afternoon. It is important to note that testosterone levels may drop below the normal range during the day in a completely normal individual. Therefore, if you are able to produce a normal serum level of testosterone during the expected peak hours of 8-10 AM, you are generally considered to be “eugonadal” (i.e, normal testicular function).
Levels of SHBG, the protein responsible for carrying the majority of bound testosterone, increase with aging, corticosteroid use, high-estrogen states, hyperthyroidism, liver disease, and malnutrition/anorexia. In contrast, levels decrease in high-androgen states (including anabolic steroid use), obesity, and hypothyroidism. At a given level of total testosterone, higher levels of SHBG result in a relatively larger fraction of “bound” (and therefore relatively less “free”) testosterone, and vice versa.
Since SHBG generally increases with age in men, age-related declines in free testosterone outpace decreases in total testosterone. There is typically a ~30% drop in total testosterone from the age of 25 to 75, while free testosterone drops by 50% or more between the same ages. Whether this represents a pathologic or physiologic condition has yet to be firmly established in the research literature.
Hypogonadism, or the Dreaded “Low T”
Hypogonadism is defined as inadequate testicular function resulting in impaired sperm production and/or impaired testosterone production. There are a wide variety of causes of hypogonadism, and the clinical features depend on:
- Age at presentation: hypogonadism presents differently in newborns, prepubertal males, and adults. Obviously, neonates and children will remain outside the scope of this article, and we will focus on hypogonadism in adult males.
- Duration & severity of testosterone deficiency: these factors play a significant role in the type, degree, and severity of symptoms at presentation.
- Genetic variations or mutations in the androgen receptor: there are a number of known mutations or genetic variants that affect the receptor’s function and/or sensitivity to testosterone. Although this is currently an active area of research with limited clinical data, it could result in clinically relevant variation in androgen sensitivity between individuals with equivalent serum testosterone levels. This could help explain the differences in thresholds for symptom onset among males as well as differences in response to therapy.
The numerous symptoms of testosterone deficiency in adult males can include:
- Fatigue, loss of “vigor”
- Decreased muscle mass
- Increased fat mass, gynecomastia
- Mood disturbance, depression, irritability, cognitive dysfunction
- Sleep disturbance
- Loss of libido, decreased spontaneous erections
- Reduced bone density, osteoporosis
- Menstrual-type hot flashes
- Infertility
- Decreased body hair, reduced need to shave, small testes
- Erectile dysfunction
Unfortunately, these symptoms are often ignored by patients and dismissed by physicians. However, it is extremely important to understand that many of these symptoms are highly non-specific. In other words, they can be caused by a number of other conditions as well, and do not guarantee a diagnosis of low testosterone. A thorough evaluation is warranted to rule out other causes like hypothyroidism, obstructive sleep apnea, anemia, other deficiencies, chronic infections (e.g., HIV), depression not due to low testosterone, medication side effects, and many others.
If the symptoms are caused by low testosterone, it is also important to note that these symptoms occur at varying levels and durations of testosterone deficiency. For example, while fatigue and loss of “vigor” are common symptoms in early or mild deficiency, widespread loss of body hair, hot flashes, and erectile dysfunction are typically manifestations of more longstanding and/or severe testosterone deficiency. So if someone is experiencing erectile dysfunction in the absence of any other symptoms causes other than testosterone deficiency should be considered. Erectile dysfunction in particular is more often a result of psychogenic, neurologic, cardiovascular, or medication-related causes than directly caused by testosterone deficiency (hence the prevalence of Viagra and Cialis prescriptions for erectile dysfunction, rather than testosterone).
Types of Hypogonadism
Primary hypogonadism refers to a problem with the testes’ ability to produce sperm and/or testosterone. It is also known as Testicular Failure or Hypergonadotropic hypogonadism, because the HPG axis' feedback results in high levels of gonadotropins (FSH/LH) in an attempt to “kick start” the unresponsive testes. These conditions are more likely to affect sperm production than testosterone production, but can present with signs of testosterone deficiency as well. Causes include various genetic syndromes, alcohol abuse, trauma, infections, chemo- or radiation therapy, and autoimmune or other chronic systemic diseases.
Secondary hypogonadism refers to a problem in the brain (hypothalamus or pituitary) affecting the hormonal stimulation and feedback mechanisms of the HPG axis, while the testes retain normal function and responsiveness. It is also known as Hypogonadotropic hypogonadism because of inadequate gonadotropin (FSH/LH) levels in response to low testosterone production.
These processes typically cause a proportionate decrease in sperm production and testosterone production. The most common causes include obesity, diabetes, alcohol abuse, medication-related (such as glucocorticoids, opiate analgesics, androgens (including anabolic steroids), estrogens), HIV infection, severe acute illness or chronic systemic disease, autoimmune disease, and "idiopathic" (i.e, "we don't know") causes.
Other, less common causes include malnutrition/malabsorption, various genetic syndromes, pituitary tumors, head trauma or surgery, radiation therapy, and infiltrative diseases. Secondary hypogonadism may also result from androgen deprivation therapy or "chemical castration" used to treat hormone-sensitive prostate cancers.
Mixed-type hypogonadism comprises most cases of "late-onset hypogonadism" in middle- and elderly men and has components of both primary (insufficient testicular response to LH stimulation) and secondary (inadequate central response to low testosterone). It shares a number of the same causative factors listed above.
Diagnosis
According to “official” endocrinology guidelines, the clinical diagnosis of hypogonadism requires characteristic signs or symptoms in combination with a decreased serum testosterone concentration. They typically recommend against widespread screening for low testosterone in patients without symptoms or risk factors for hypogonadism. Most physicians will therefore be hesitant to measure testosterone levels simply based on patient request, because it is often impossible to interpret the significance of a “low” result in the absence of symptoms. As discussed above, the presence and severity of symptoms may be related to the duration and degree of deficiency compared to their individual “optimal” level, as well as genetic variations in testosterone sensitivity. Therefore, while some males may be completely asymptomatic despite a level in the 100s, other reports have described symptomatic patients in the mid 300s ng/dL.
So, what level of testosterone is really “low”? Unfortunately there is no perfect cut-off that perfectly separates normal from hypogonadal, just in the same way that there is no consistent threshold for when symptoms start to appear in all men.
There have been numerous suggestions from official organizations as to what level of 8-10 AM total testosterone should be considered “low” and therefore prompt treatment. Most organizations’ recommendations fall somewhere between the mid-200s ng/dL and the low-to-mid 300s ng/dL, independent of age. Others argue for stratifying reference ranges by age range, although there is limited evidence for this approach as well. Given the discussion of genetic variation in androgen receptor sensitivity, it has been suggested that in the future, a strict threshold value “is likely to be replaced by a continuum spanned by genetics as well as symptom specificity.”
However, an argument can also be made that the variety of nonspecific symptoms of hypogonadism might go unnoticed for a long period of time as people become accustomed to their “new normal.” They may never specifically complain of classic hypogonadal symptoms, while having potential for significant improvement in quality of life with optimal levels. Unfortunately, there is very little solid data on the matter to provide guidance in clinical decision making, and screening therefore remains highly controversial.
Typical laboratory measures might include Total, Free or Bioavailable Testosterone, SHBG, FSH, LH, TSH, and Prolactin. It should be noted that Free Testosterone assays are often unreliable unless specifically performed through a specialized lab technique, and therefore a measurement of Bioavailable Testosterone (which includes both albumin-bound and free testosterone) is the preferred measure when indicated.
Step 1
If you are symptomatic or have known risk factors for hypogonadism (listed above), start with a Total Testosterone measurement drawn between 8 and 10 AM (assuming a “normal” daily schedule) to catch a peak level for the day. This measurement should be performed in a fasting state since glucose ingestion can acutely suppress testosterone levels and potentially produce a false positive result. If you have symptoms, you should probably have a number of other studies performed at this stage as well, potentially including (but not limited to) a medication review, basic blood counts and chemistries, thyroid function, vitamin D level, depression screening, sleep evaluation, HIV screening, and perhaps others depending on your individual situation.
There are also several validated screening questionnaires (e.g., Androgen Deficiency in Aging Men, Androtest, etc.) with reasonable sensitivity (i.e, can help "rule out" hypogonadism), but given the multitude of nonspecific symptoms associated with hypogonadism, they generally have limited specificity to help "rule in" the diagnosis. However, they may still be useful to monitor symptoms and long-term response to therapy.
Step 2
If your initial total testosterone level was measured properly (8-10 AM, fasting) and showed a low result, the level should be rechecked at least once more for confirmation given high day-to-day variability. This next step should also include FSH and LH measurement to differentiate primary from secondary hypogonadism, if present.
If you are overweight/obese, older, or if the first measurement was low-normal or otherwise does not correspond to your symptoms, consider checking a Bioavailable Testosterone level or SHBG in this step as well. In these situations, a total testosterone measurement may be falsely low due to a low SHBG level, while the amount of free/bioavailable testosterone is actually normal. In this case, treatment would not be necessary or beneficial. Conversely, a low bioavailable testosterone fraction or a normal/elevated SHBG level in this situation could be consistent with hypogonadism.
Step 3
If a properly measured total testosterone level is low on at least two occasions, look to the gonadotropin measurements to localize the source of the problem. In this situation, elevated FSH and LH (i.e, hypergonadotropic hypogonadism) points to the testes as the source. This requires further evaluation, likely including genetic testing for a specific diagnosis. However, this is a less likely situation among our adult male demographic.
A more likely result will show inappropriately normal or low FSH and LH for the degree of testosterone deficiency (i.e, hypogonadotropic hypogonadism). This points to the brain (hypothalamus, pituitary) as the primary source of the problem. This situation also requires further evaluation, including measurement of prolactin and thyroid hormone (if not measured already), 8 AM cortisol level, iron panels, and – if severely low (e.g., less than 150 ng/dL), or symptoms including headache or visual changes – an MRI of the brain might be performed to rule out a pituitary tumor as the cause. The remaining causes of secondary hypogonadism discussed above should also be considered.
Indeterminate FSH/LH levels may require specialized testing by an endocrinologist (e.g., GnRH, clomiphene, or hCG stimulation testing) and are beyond the scope of this article.
Treatment
Given the ambiguity regarding testosterone level “cutoffs,” there are no specific guidelines on exactly who is a candidate for testosterone replacement therapy (TRT). After making the diagnosis of clinical hypogonadism, the decision to proceed with TRT should involve a discussion with your doctor.
Initiating treatment in the setting of untreated sleep apnea, heart failure, hematocrit over 54%, or PSA over 4 ng/mL is not recommended. And although fears of side effects and complications are often blown out of proportion, they are still possible, and since treatment is often (but not always) a lifelong endeavor, the decision to initiate treatment should not be taken lightly.
It is also very important to understand that not everyone with “Low T” requires immediate testosterone replacement therapy. Several of the most common causes of hypogonadism (e.g., obesity/diabetes, alcohol abuse, medication-related, sleep apnea) are reversible with simple interventions such as improved diet and exercise, reduction of alcohol consumption, discontinuation or switching of medications, or simple use of a CPAP machine. It is extremely common for testosterone levels to promptly normalize once these issues are addressed, so consider this before pushing your doctor for a prescription.
However, if TRT is planned, goals of therapy include:
- Restoration of normal physiologic levels
- Restore sexual function, libido, “vigor,” energy, and/or mood
- Improvement in body composition and strength
- Optimization of bone density, prevention of osteoporosis and fractures
- Induction or maintenance of virilization (if applicable)
Initial effects of testosterone replacement therapy on most of these parameters begins within 3-6 weeks, but may take up to 6-12 months (or years, in the case of bone density) to maximize and stabilize.
Treatment Options and Associated Medications
Injectables, most commonly Testosterone enanthate or Testosterone cypionate esters in oil suspension, are the preferred modes of treatment. Usual starting dose is around 100 mg once per week and titrated as needed for physiologic levels. Benefits include low cost and extensive clinical experience with these options, while drawbacks include the need for intramuscular injections. Physicians occasionally prescribe slightly higher doses (e.g. 200-300 mg) administered every two to three weeks; however, this often results in unpleasant fluctuations in symptoms as the initial injection causes a very rapid rise (often to supraphysiologic levels) followed by a decline to very low levels by the time the next injection is due. Therefore, continued or fluctuating symptoms while using injectable testosterone esters should prompt an evaluation of symptom patterns, dosage, and the administration protocol. Occasionally adjusting the dosing frequency by a day or two may be all that's needed to fix the problem.
Transdermal products (gels and patches) are among the most popular products, mainly driven by pharmaceutical industry promotion and convenience. Typical dosing depends on the specific product, but transdermals are often dosed 1-2 times per day. They often result in slightly lower, but steadier levels compared to injection therapy. Daily administration theoretically helps to maintain the physiologic diurnal rhythm, although specific benefits of this are unknown. Gels are significantly more expensive than injections, and have a risk of skin-to-skin transfer with others (including women and children) through touch.
Implantable pellets slowly release testosterone into the blood over a 3-6 month time period. They are also significantly more expensive than injections, and require repeated minor surgical procedures for implantation and extraction every 3-6 months. However, once in place, they are more convenient than other products due to the controlled release with low-frequency of “re-dosing”.
Other, newer products include nasal gels and lozenges, which have increased dosing frequencies of two to three times daily. There is limited clinical experience with these options, and this dosing frequency and local side effects are inconvenient for most men.
Oral products such as methyltestosterone are no longer available on the market due to dangerous side effects including hepatotoxicity, dyslipidemia, and liver cancer. Other oral anabolic agents such as oxandrolone, methandrostenolone, and ostarine are not used for the treatment of hypogonadism.
Clomiphene citrate is an oral medication that works by blocking estrogen receptors in the hypothalamus, interfering with the natural feedback mechanisms to produce an increase in FSH/LH levels that stimulate the testes. Although intended for use in women to stimulate ovulation, it is sometimes used off-label in men to increase testosterone levels while maintaining fertility, as well as to help restore fertility after stopping TRT or anabolic steroid use. Although it is effective in increasing testosterone levels, men generally report poor improvement in their symptoms as well as undesirable emotional side effects.
hCG and hMG are human hormones that mimic the activity of LH and FSH on the testes, and can be used as injections 2-3 times per week to stimulate sperm production in infertile males. They are also used in an off-label fashion alongside (or after stopping) TRT to maintain or restore testicular volume and fertility since TRT (or AAS use) can cause testicular atrophy.
Anti-estrogens including Tamoxifen, Anastrozole, Letrozole, and Exemestane are oral medications used off-label to manage signs and symptoms resulting from estrogen excess.
Adverse effects and Monitoring
By the end of the first month of TRT, a serum testosterone level should be checked to ensure therapeutic levels. For injection therapy, some physicians check a level mid-way between injections, while others prefer a “trough” level drawn immediately prior to injection. Either is a reasonable approach, and adjustments in dose and frequency can be made to target and maintain physiologic levels, reduce hypogonadal symptoms, and minimize side effects.
Estradiol (E2) levels do not typically need to be monitored in most individuals, but could be checked in obese individuals, those using high doses of testosterone, or in those experiencing signs or symptoms of estrogen excess. Such symptoms include gynecomastia, sore/itching nipples, bloating, and mood/libido issues. If elevated E2 levels are confirmed, the TRT dose can be adjusted, or aromatase inhibitors can be used in an off-label fashion to target a range of 20-30 pg/mL, with caution to avoid oversuppression.
During the first year of treatment, monitoring should also include hematocrit levels, particularly in injection TRT users. It has been recommended to stop therapy if the hematocrit (a measure of red blood cell volume) rises over 54%, although this specific cutoff has not been well-studied and the available data is controversial regarding the risk of adverse cardiovascular events at this specific level. Alternatively, hematocrit can be lowered through regular blood donation, and many individuals will also take 81 mg aspirin daily to further reduce risk.
Adverse changes in serum lipids are relatively uncommon with traditional dosing of TRT targeting physiologic levels, whereas it is far more common when testosterone is abused to achieve supraphysiologic levels or when using oral anabolic-androgenic steroids. To date, the evidence of increased cardiovascular risk (including heart attack and stroke) from TRT has been inconsistent, and therefore controversial. The FDA nonetheless requires a warning about the possibility of increased cardiovascular risk from testosterone therapy, so if you are already at particularly high cardiovascular risk (e.g., prior heart attack or stroke, active smoking, strong family history), caution with TRT is advised.
Finally, prostate monitoring (including prostate symptom screening, prostate exam, and PSA level) is recommended. Potentially concerning increases in PSA concentration include an increase of 1.4 ng/ml over a baseline measurement, or a rate of increase (“PSA velocity”) of greater than 0.4 ng/ml per year for at least two years. This is due to concern over testosterone therapy stimulating prostate growth and, potentially, cancer development. However, the fear of causing prostate cancer is strictly theoretical; to date, there has been no clear evidence showing TRT to increase the risk of prostate cancer.
Other adverse effects may include alopecia (in susceptible individuals), acne/oily skin, night sweats, exacerbation of obstructive sleep apnea, infertility, and blood clots (particularly in those with a history of clotting disorder). Of course, all adverse effects are far more common in individuals abusing testosterone to achieve supraphysiologic levels.
Myths & Misconceptions
Finally, we should dispose of a few myths and misconceptions about testosterone. Men ask all the time what supplements they can use to “increase their testosterone naturally.”
Unfortunately, the vast majority of supplements advertised for the purpose of increasing testosterone have absolutely no such effect. This includes commonly encountered products such as Tribulus terrestris, Horny Goat Weed, stinging nettle and a number of others. A few supplements, including D-aspartic acid, dehydroepiandrosterone (DHEA), and possibly Fenugreek may improve testosterone levels, but these are relatively small and often temporary effects that are simply not worth the time or money.
Additionally, the fear of soy protein consumption reducing testosterone levels (or increasing estrogen) is similarly unfounded. While certain soy isoflavones have been shown to have weakly estrogenic and antiandrogenic activities in lab research, the current data does not show any significant effect of soy protein consumption on serum testosterone or estrogen levels in men.
Other silly and unfounded strategies include routinely taking ice-cold showers and abstinence from sex in order to boost testosterone levels. These have no supporting evidence for their efficacy, and purposefully abstaining from sex for prolonged periods of time in order to increase testosterone seems rather counterproductive if the goal is to improve one’s quality of life.
Fortunately, there are several well-established lifestyle measures that improve testosterone levels naturally. Although often easier said than done, the following factors should ideally be optimized prior to consideration of starting TRT:
- Losing excess bodyfat
- Reducing use of alcohol, marijuana, and opiate pain medications
- Treating obstructive sleep apnea and other underlying medical issues
- Improve sleep quality
- Healthy diet, including adequate intake of total calories and saturated fats
- Resistance training (e.g., heavy barbell training)
- Reduce stress
- Safe sexual activity
- Ensuring adequate vitamin D levels
Optimal testosterone levels are crucial for a number of physiologic functions in the human body, and symptomatic hypogonadism can be an under-recognized yet debilitating condition at any age. It is of unique concern in the aging population, where anabolic resistance and sarcopenia are major detractors from quality of life and risk factors for mortality. Symptoms potentially due to hypogonadism should undergo medical evaluation; however, lifestyle factors should be the primary target for interventions before testosterone replacement therapy is considered or initiated. However, properly managed testosterone replacement therapy is generally safe, well tolerated, and can provide immeasurable benefits in quality of life.
Thanks to CJ Gotcher for his assistance in editing this article.
Disclaimer: The medical information in this article is provided as an information resource only, and is not to be treated as advice, or used/relied on for any diagnostic or treatment purposes. This information does not create any patient-physician relationship, and should not be used as a substitute for professional diagnosis and treatment. Please consult your healthcare provider before making any healthcare decisions, or for guidance about a specific medical condition. The author(s) expressly disclaim responsibility, and shall have no liability, for any damages, loss, injury, or liability whatsoever suffered as a result of your reliance on the information contained in this article.
References
AACE Hypogonadism Guidelines, Endocr Pract. 2002;8(No. 6)
Arver S, Lehtihet M. Current Guidelines for the Diagnosis of Testosterone Deficiency. Front Horm Res. 2009;37:5-20.
Lunenfeld B., et al. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male. 2015 Mar;18(1):5-15.
Davey RA et al. Androgen Receptor Structure, Function and Biology: From Bench to Bedside. Clin Biochem Rev. 2016 Feb;37(1):3-15.
Dandona, P et al. A Practical Guide to Male Hypogonadism in the Primary Care Setting. International Journal of Clinical Practice 64.6 (2010): 682–696.
Hamilton-Reeves et al. Clinical studies show no effects of soy protein or isoflavones on reproductive hormones in men: results of a meta-analysis. Fertility and Sterility, Volume 94, Issue 3, 997 – 1007.
Snyder et al. Clinical features and diagnosis of male hypogonadism. UpToDate. Accessed 11/14/16.
Snyder et al. Testosterone treatment of male hypogonadism. UpToDate. Accessed 11/14/16.



























